Product lifecycle management software to help bring products to market quickly, profitably and in compliance
San Jose, CA — July 15, 2003 — Agile Software Corp. today announced the launch of AgileMD, a product lifecycle management (PLM) solution designed specifically for the medical device industry.
Agile said companies in the medical device industry are currently using its products to achieve such PLM business objectives as improving cycle time up to 60 percent, reducing operating costs by 25 percent and materials costs by 5 percent, satisfying FDA requirements for Corrective Action/Preventative Action (CAPA) management, and complying with FDA Title 21 CFR Part 11, which prescribes the accepted use of electronic records and signatures.
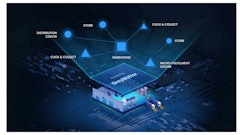
The PLM solution is created to manage the medical device product record from research and development, clinical trials, regulatory approval, new product introduction, market acceptance, to product phase-out.
Gisela Wilson, director of product life-cycle management solutions, IDC, said, "This market depends on its ability to rapidly innovate its product offerings, and at the same time, support rigorous FDA requirements for electronic documentation. IDC believes AgileMD can help medical device and instrumentation manufacturers develop and maintain competitive product portfolios."