FDA is launching a pilot program that will ensure greater accountability for the U.S. pharmaceutical drug supply chain.
According to European Pharmaceutical Review, the pilot will test innovative and emerging approaches for enhances tracing and verification of prescription drugs in the U.S.. It also aims to reduce diversion of drugs distributed domestically, hoping to keep counterfeit drugs from entering the supply chain.
“As part of our ongoing efforts to protect our nation’s drug supply, today, we’re giving industry an opportunity to test new technologies that can help spur greater accountability for participants in the supply chain and improve our ability to trace prescription drugs at every point in the distribution chain,” said FDA Commissioner Scott Gottlieb in a statement. “We recognize that tracking and tracing products is critical to industry’s ability to detect and remove potentially dangerous drugs from the drug supply chain. This pilot is one of many steps we’re taking to foster innovative ways to improve the security of the drug supply.”
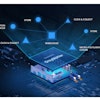
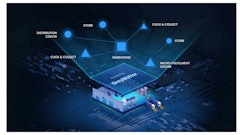
In addition to tracing the drugs, the program is also intended to assist the FDA and members of the pharmaceutical distribution supply chain in the development of the interoperable electronic system that will be established by 2023.