*Sponsored by Movilitas*
The World Health Organization (WHO) has estimated that 10% of global pharmaceutical commerce involves counterfeit drugs. In Europe alone, it is estimated that the pharmaceutical industry was losing €9.6 billion in sales due to counterfeits during the period of 2012-2016, which represents 3.9% of total sales.
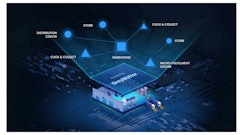
Biotechnology, drug, food manufacturers, wholesalers and other stakeholders are vital to the health of the global population. However, these companies often operate under strict regulations to ensure the safety and quality of drugs, food and other goods at each level, from raw material to consumer distribution. Any disruption in the supply chain can have unforeseen ripple effects across the system.
End-to-end traceability is the basis for enhanced consumer protection from falsified or damaged products. With the knowledge about the physical location of a particular product within a supply chain at any point in time, you can better detect counterfeiting incidents, minimize the delivery of damaged goods and reduce product diversion.
The regulatory landscape is ever-changing as well as expanding into new industries, such as medical devices, high tech, chemicals and automotive. Any organization selling products into these regulated markets should expect requirements to continuously evolve. Additionally, there can be traceability rules for safety and sustainability initiatives. Let’s have a look at a sample of regulations companies need to comply with.